When a COVID-19 vaccine becomes available, it will be in short supply and rationed by the federal government.
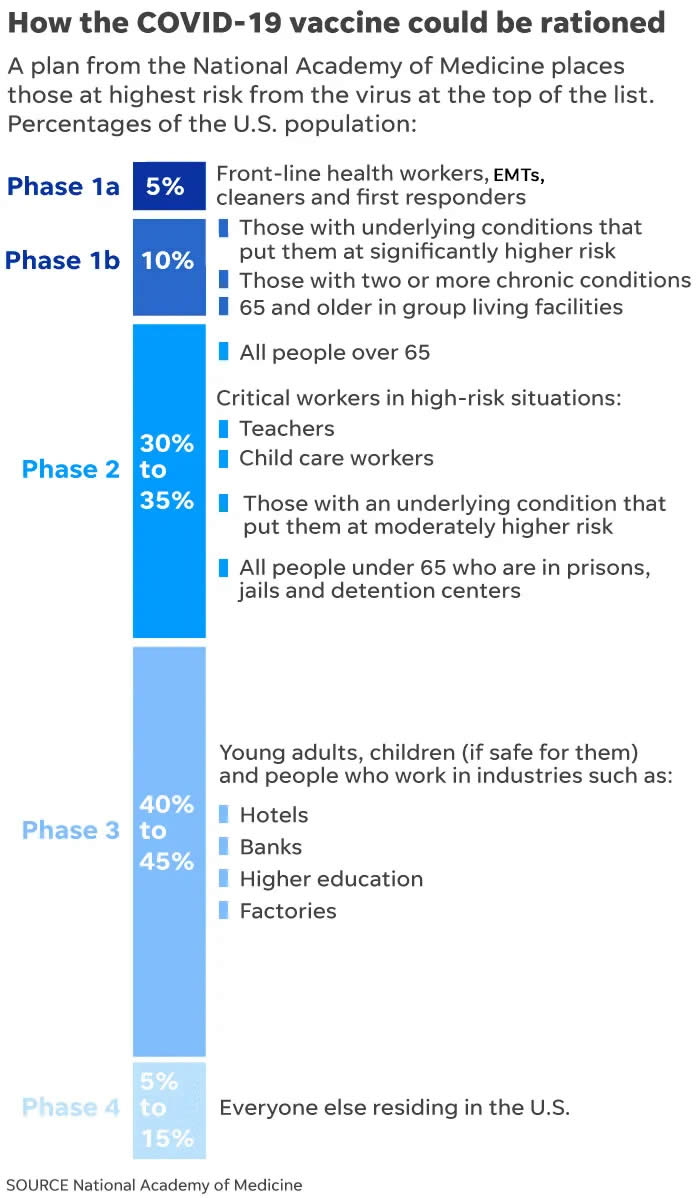
The National Academies of Sciences, Engineering, and Medicine has outlined a plan for fair distribution that’s being used as a framework by the Centers for Disease Control’s Advisory Committee on Immunization Practices, which soon will make the final decision on when certain groups of Americans will have access to vaccine.
The process could start very soon. The first COVID-19 vaccine is anticipated to be authorized by the Food and Drug Administration within the next month, with distribution to start in no more than 24 hours to every state in the union. A second vaccine could be authorized two weeks later. Enough vaccine for 20 million people is expected to be available in December, with more coming in 2021.
A “jumpstart group” will be first in line — people who risk their lives to care for the sick and keep society safe. That includes frontline health care workers, first responders, cleaners and ambulance drivers. There are three other priority groups before the general population. To reach everyone could take up to a year.
Here’s the tentative rollout plan: